ABRASIVES
These are substances or chemicals that depend on their rubbing or scratching action to clean dirt and grit from hard surfaces. They are used to remove very stubborn stains on various surfaces.
Abrasives are classified as:
Talc Calcite Feldspar Diamond
Scale of hardness
Medium abrasives – These include rotten stone, salt, scouring powder and scouring paste. Scouring powders are made up of fine particles of pumice mixed with a soap/detergent, an alkali and a little bleach.
Hard/coarse abrasives – These include bath bricks, sand paper, pumice, steel wool and emery paper.
Glass paper, calcite, sand paper, fine ash, emery powder and paper, jeweler’s rouge, powdered pumice, precipitated whiting (filtered chalk), feldspar, ground limestone, sand, carborundum, steel wool and nylon scourers are some commonly used abrasives.
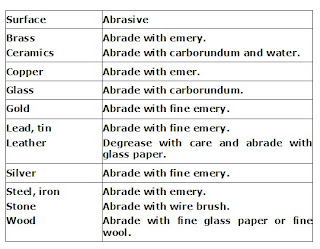
Abrasives are usually not used alone in cleaning agents. For example, cream or paste meant for cleaning utensils contains about 80% of finely ground limestone, along with other substances such as bleachers, anionic surfactants, alkaline builders and perfumes. The use of various abrasive agents for cleaning different surfaces is summarized in above table
MANWAL